In new level of stupid, RFK Jr.’s anti-vaccine advisors axe MMRV recommendation
The vote to strip the recommendation came after a day of inept discussion.
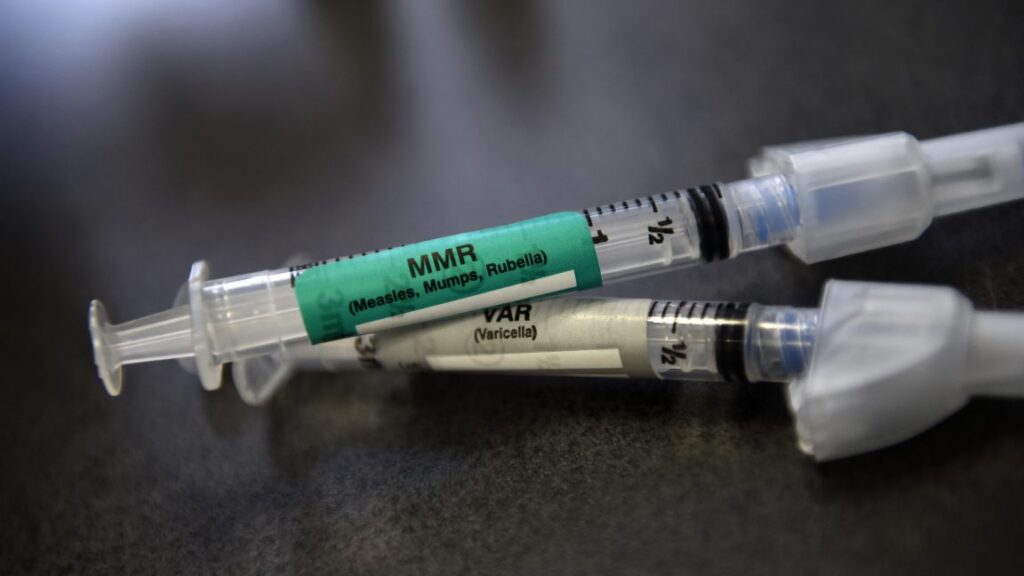
An MMR and VAR vaccine ready for a pediatric vaccination at Kaiser Permanente East Medical offices in Denver in 2015. Credit: Getty | Joe Amon
The panel of vaccine advisors hand-selected by anti-vaccine activist Robert F. Kennedy Jr. voted on Thursday to change the federal vaccine recommendations for children, removing safe, well-established vaccine doses from current schedules and realizing Kennedy’s anti-vaccine agenda to erode federal vaccine policy and sow distrust.
Specifically, the panel—the Advisory Committee on Immunization Practices (ACIP)—voted to remove the Centers for Disease Control and Prevention’s previous recommendation for use of a measles, mumps, rubella, varicella (chickenpox) MMRV combination vaccine for children under 4 years old.
The context
In June, Kennedy fired all 17 highly qualified, highly vetted members of ACIP and quickly replaced them with seven questionable members, who largely did not have subject matter expertise. Moreover, many of them have clearly expressed anti-vaccine rhetoric and skepticism about pandemic responses and COVID-19 vaccines. At least two new members have been paid witnesses in trials against vaccine makers, a clear conflict of interest. Earlier this week, Kennedy added five additional members, who raise the same anti-vaccine concerns as the first group.
In the meeting today—the first of two all-day meetings—members made clear their inexperience and lack of expertise in evaluating vaccine policy. They asked basic questions about study data and analysis—such as asking what a “low confidence” designation means—and claimed CDC presentations lacked critical data when, in fact, a CDC scientist had just presented the exact data in question.
The first half of the day focused on the MMRV vaccine, while the second half focused on a newborn dose of the hepatitis B (hep B) vaccine. A vote was initially scheduled for that vaccine today, too, but was postponed after the panel decided to change the wording of the voting question. They meet again tomorrow to vote on the hep B recommendation as well as recommendations for this year’s COVID-19 vaccine. Ars Technica will have coverage of the second half of the meeting tomorrow, along with a report on the hepatitis B discussion today.
MMRV vaccine change
For the MMRV vaccine, the panel rehashed an issue that vaccine experts had thoroughly examined years ago. Currently, the CDC recommends children get vaccinated against measles, mumps, rubella, and varicella (chickenpox) twice—one dose at 12 to 15 months, and a second dose between the ages of 4 and 6 years.
In 2005, the Food and Drug Administration approved a combo shot for all four—the MMRV vaccine—which provided an alternative to the previous method of giving an MMR vaccine dose (against measles, mumps, and rubella) plus a separate varicella vaccine dose at the same time. (This vaccination strategy is shorthanded as MMR + V.) Thus, the MMRV combo shot meant one fewer shot for children. But, in 2008, post-market data suggested that the MMRV shot might have a slightly higher risk of causing febrile seizures (seizures associated with fevers), which is a very low risk with the MMR + V separate shots.
Febrile seizures are a somewhat common reaction in young children; this type of seizure almost entirely occurs in children under age 5 years, most often striking between 14 and 18 months. The seizures are short, usually less than a minute or two, and they can be caused by essentially anything that can cause a fever—ear infections, vaccines, the flu, etc. For parents, a febrile seizure can be very scary and lead them to bring their child to a doctor or hospital. However, febrile seizures are almost always harmless—the prognosis is “excellent,” as CDC staff experts noted. Nearly all children fully recover with no long-term problems. By age 5, up to 5 percent of all children have had a febrile seizure at some point, for some reason.
Low risks
In post-market studies of the MMRV vaccine, it was very clear that a slightly increased risk of febrile seizures was only linked to the first dose (given at 12 to 15 months, not the second, given at 4 to 6 years). In studies of over 400,000 children, data found that the risk of a febrile seizure after a first-dose MMRV vaccine was 7 to 8.5 seizure cases for every 10,000 vaccinations. That’s compared to 3.2 to 4.2 seizure cases in 10,000 vaccinations with MMR + V. In all, a first-dose MMRV vaccine had about one additional febrile seizure per 2,300 to 2,600 children vaccinated compared with MMR + V.
In 2009, CDC vaccine experts reviewed all the data and updated the vaccine recommendation. They maintained that MMRV and the MMR+V vaccinations are still both safe, effective, and recommended at both vaccination time points. But, they added the nuance that there is a preference (or a default, basically) for using the MMR + V shots for the first dose, unless a parent expressly wanted the MMRV vaccine for that first dose. This skirted the slightly increased risk of febrile seizure in young children, without entirely taking away the option if a parent prioritized fewer jabs and wanted the MMRV. For the second dose, again, both MMRV and MMR + V are options, but the CDC stated a preference for the one-shot MMRV.
Since then, about 85 percent of vaccinated children have gotten MMR + V for their first dose shots, with the other 15 percent getting the MMRV vaccine.
Inept discussion
In the discussion today, Kennedy’s members seemed to have little grasp of the issue at hand and the clinical significance of febrile seizures generally. They continued to circle back to unfounded concerns about febrile seizures and fringe theories about potential long-term effects.
Cody Meissner, a pediatric professor at Dartmouth’s Geisel School of Medicine who has served on ACIP in the past—arguably the most qualified of Kennedy’s new lineup—was bewildered at why the committee was rehashing the issue addressed years ago. “This discussion is really a déjà vu for me,” he said. Yet, while Meisner felt the issue was settled and pediatricians were well-equipped to calm parents’ fears about febrile seizures, the other members could not be swayed. They claimed, without evidence, that parents of children who have febrile seizures after a vaccine would be less likely to get future vaccines.
As the committee seemed to be leaning toward removing the recommendation for MMRV for the first dose, Jason Goldman, president of the American College of Physicians, who attended the meeting as a liaison, pushed back strongly. He pointed out that—as with the last time Kennedy’s ACIP met—they were not following the standard framework for making and changing recommendations.
“Are we going to have a thoroughly vetted evidence-to-recommend framework presentation that looks at all the harms benefits, acceptability, feasibility—with input from practicing clinicians and liaisons in order to make an informed decision?” Goldman asked. “I would argue that this recommendation is going to create more confusion among the public.”
Goldman noted that if the committee rescinds the recommendation for MMRV for children under 4, the shot would no longer be covered by the Vaccines for Children (VFC) Program, a federal program for Medicaid-eligible and under- or uninsured kids, which covers about half of American children.
“And finally, you are taking away the choice of parents to have informed consent and discussion with their physician on what they want to do for the health and benefit of their children,” Goldman said. “So, I urge this committee not to change the recommendations if they truly want to give the power to the parents to decide what is best for their child and allow them to make the choice in consultation with their physicians.”
Voting confusion
In the end, Kennedy’s panel voted 8–3 (with one abstention) to not recommend MMRV for children under age 4, meaning the MMRV vaccine could potentially no longer be available for some children under age 4. Private insurance companies are required to cover ACIP-recommended vaccines, so this move strips the recommendation and that coverage requirement.
But, anticipating such a change, AHIP, a trade organization representing insurance companies, put out a statement earlier this week suggesting that they would still cover the MMRV vaccine for children under 4, even if it’s not required.
“Health plans will continue to cover all ACIP-recommended immunizations that were recommended as of September 1, 2025, including updated formulations of the COVID-19 and influenza vaccines, with no cost-sharing for patients through the end of 2026,” the statement reads.
But, there’s more: In a second vote today, ACIP voted 8–1 (with three abstentions) against changing VFC coverage for MMRV. Therefore, the VFC program will continue to cover MMRV vaccines for children under age 4. This is a split from standard policy that is likely to spur confusion, because VFC typically goes with ACIP recommendations. Also, Medicaid’s Children’s Health Insurance Program (CHIP) has to follow the ACIP vaccine recommendation and thus will no longer cover MMRV for children under age 4 covered by CHIP.
One of the abstentions on the VFC coverage vote was Meissner, who didn’t want to strip the recommendation or the VFC coverage but was entirely confused by how this would work in practice.
Beth is Ars Technica’s Senior Health Reporter. Beth has a Ph.D. in microbiology from the University of North Carolina at Chapel Hill and attended the Science Communication program at the University of California, Santa Cruz. She specializes in covering infectious diseases, public health, and microbes.
In new level of stupid, RFK Jr.’s anti-vaccine advisors axe MMRV recommendation Read More »