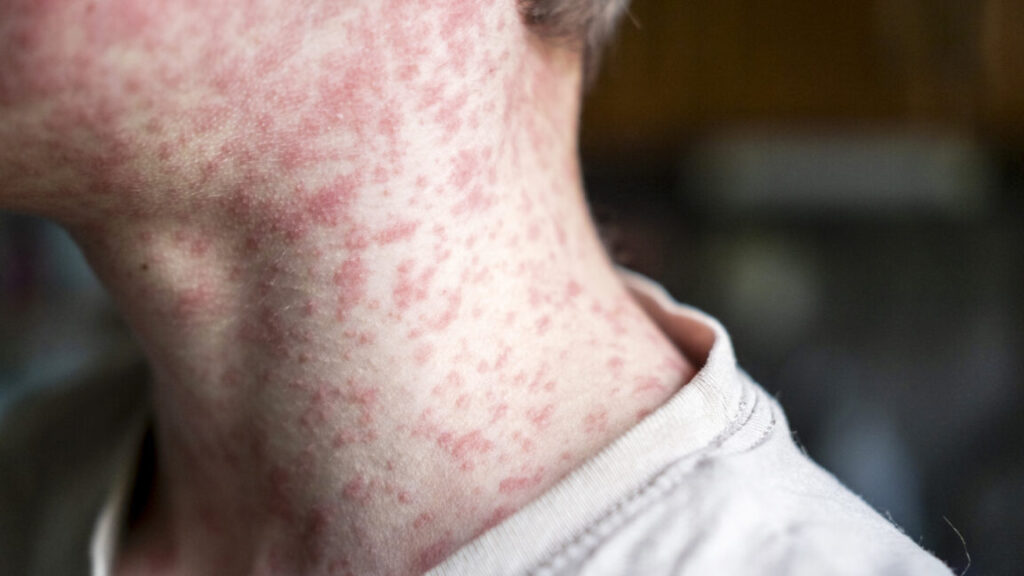
Canada fought measles and measles won; virus now endemic after 1998 elimination
“This loss represents a setback, of course, but it is also reversible,” Jarbas Barbosa, director of PAHO, said in a press briefing Monday.
Call to action
Barbosa was optimistic that Canada could regain its elimination status. He highlighted that such setbacks have happened before. “In 2018 and 2019, Venezuela and Brazil temporarily lost their elimination status following large outbreaks,” Barbosa noted. “Thanks to coordinated action by governments, civil society, and regional cooperation, those outbreaks were contained, and the Region of the Americas regained its measles-free status in 2024.”
On Monday, the Public Health Agency of Canada released a statement confirming that it received notification from PAHO that it had lost its measles elimination status, while reporting that it is already getting to work on earning it back. “PHAC is collaborating with the PAHO and working with federal, provincial, territorial, and community partners to implement coordinated actions—focused on improving vaccination coverage, strengthening data sharing, enabling better overall surveillance efforts, and providing evidence-based guidance,” the agency said.
However, Canada isn’t the only country facing an uphill battle against measles—the most infectious virus known to humankind. Outbreaks and sustained spread are also active in the US and Mexico. To date, the US has documented at least 1,618 measles cases since the start of the year, while Mexico has tallied at least 5,185. Bolivia, Brazil, Paraguay, and Belize also have ongoing outbreaks, PAHO reported.
As of November 7, PAHO has collected reports of 12,593 confirmed measles cases from 10 countries, but approximately 95 percent of them are in Canada, Mexico, and the US. That total is a 30-fold increase compared to 2024, PAHO notes, and the rise has led to at least 28 deaths: 23 in Mexico, three in the United States, and two in Canada.
The PAHO used Canada’s loss as a call to action not just in the northern country, but the rest of the region. “Every case we prevent, every outbreak we stop saves lives, protects families, and makes communities healthier,” Barbosa said. “Today, rather than lamenting the loss of a regional status, we call on all countries to redouble their efforts to strengthen vaccination rates, surveillance, and timely response to suspected cases—reaching every corner of the Americas. As a Region, we have eliminated measles twice. We can do it a third time.”
Canada fought measles and measles won; virus now endemic after 1998 elimination Read More »