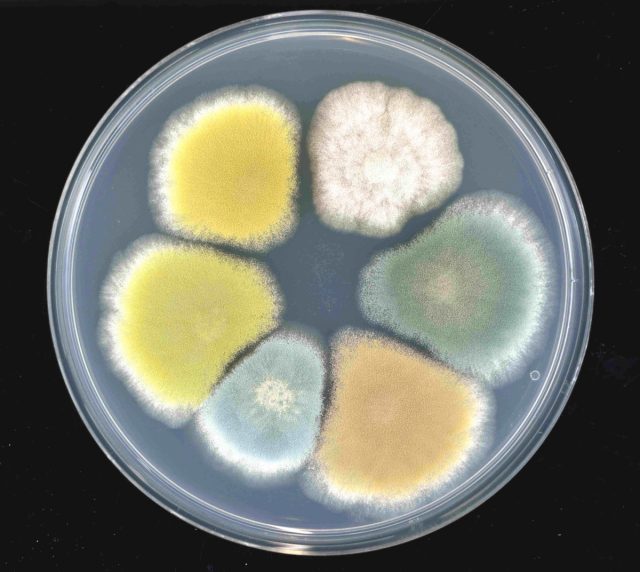
Scientist pleaded guilty to smuggling Fusarium graminearum into US. But what is it?
Even with Fusarium graminearum, which has appeared on every continent but Antarctica, there is potential for introducing new genetic material into the environment that may exist in other countries but not the US and could have harmful consequences for crops.
How do you manage Fusarium graminearum infections?
Fusarium graminearum infections generally occur during the plant’s flowering stage or when there is more frequent rainfall and periods of high humidity during early stages of grain production.
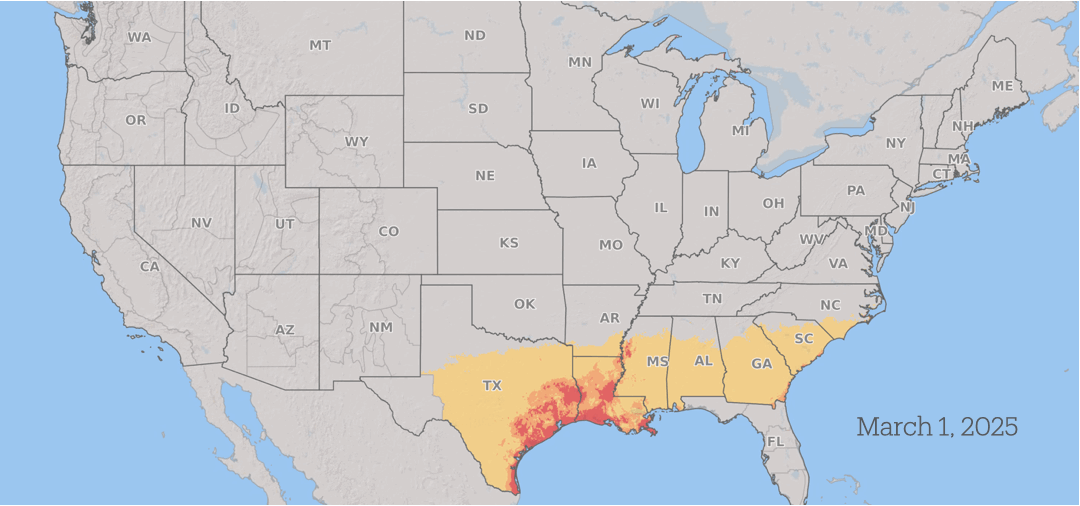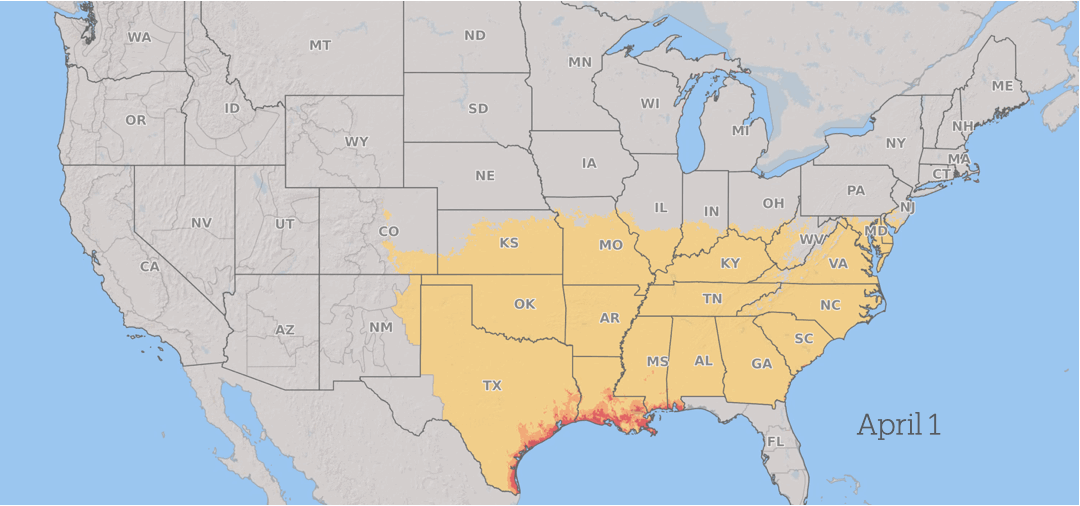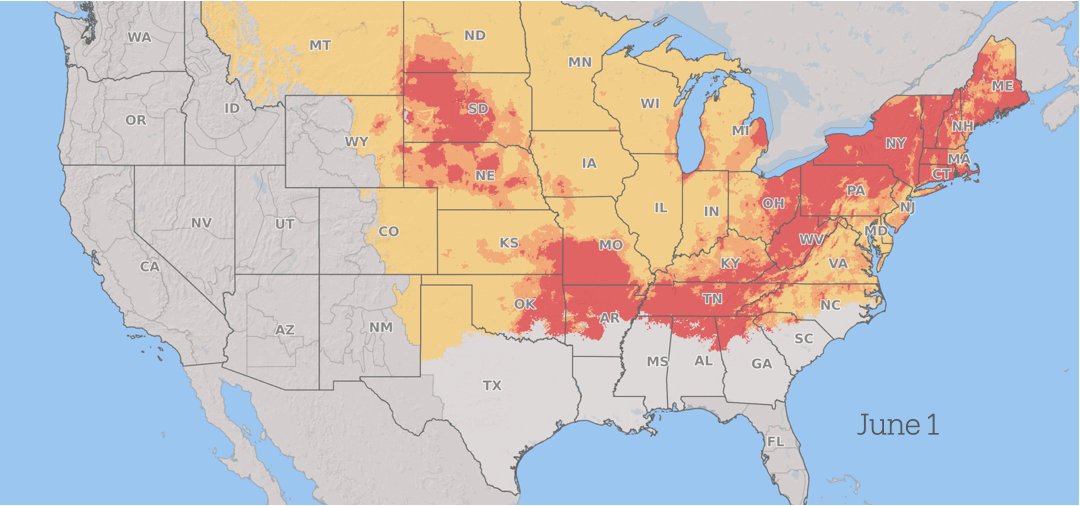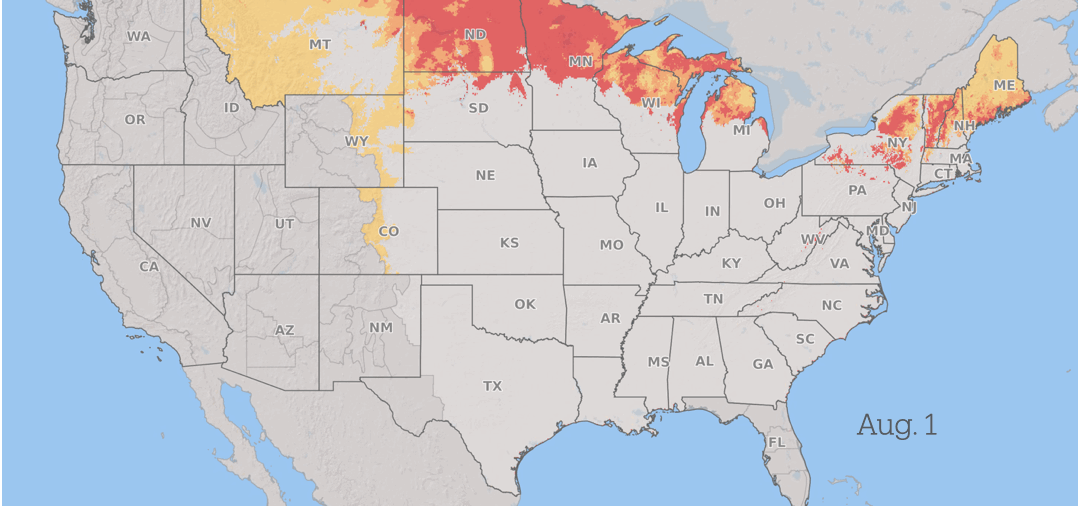
How Fusarium graminearum risk progressed in 2025. Yellow is low risk, orange is medium risk, and red is high risk. Fusarium Risk Tool/Penn State
Wheat in the southern US is vulnerable to infection during the spring. As the season advances, the risk from scab progresses north through the US and into Canada as the grain crops mature across the region, with continued periods of conducive weather throughout the summer.
Between seasons, Fusarium graminearum survives on barley, wheat, and corn plant residues that remain in the field after harvest. It reproduces by producing microscopic spores that can then travel long distances on wind currents, spreading the fungus across large geographic areas each season.
In wheat and barley, farmers can suppress the damage by spraying a fungicide onto developing wheat heads when they’re most susceptible to infection. Applying fungicide can reduce scab and its severity, improve grain weight, and reduce mycotoxin contamination.
However, integrated approaches to manage plant diseases are generally ideal, including planting barley or wheat varieties that are resistant to scab and also using a carefully timed fungicide application, rotating crops, and tilling the soil after harvest to reduce residue where Fusarium graminearum can survive the winter.
Even though fungicide applications may be beneficial, fungicides offer only some protection and can’t cure scab. If the environmental conditions are extremely conducive for scab, with ample moisture and humidity during flowering, the disease will still occur, albeit at reduced levels.
Fusarium Head Blight with NDSU’s Andrew Friskop.
Plant pathologists are making progress on early warning systems for farmers. A team from Kansas State University, Ohio State University, and Pennsylvania State University has been developing a computer model to predict the risk of scab. Their wheat disease predictive model uses historic and current environmental data from weather stations throughout the US, along with current conditions, to develop a forecast.
In areas that are most at risk, plant pathologists and commodity specialists encourage wheat growers to apply a fungicide during periods when the fungus is likely to grow to reduce the chances of damage to crops and the spread of mycotoxin.
Tom W. Allen, associate research professor of Plant Pathology, Mississippi State University. This article is republished from The Conversation under a Creative Commons license. Read the original article.
Scientist pleaded guilty to smuggling Fusarium graminearum into US. But what is it? Read More »