Homeopathic company refuses to recall life-threatening nasal spray, FDA says
Dangerous —
Consumers should stop using SnoreStop, FDA says.
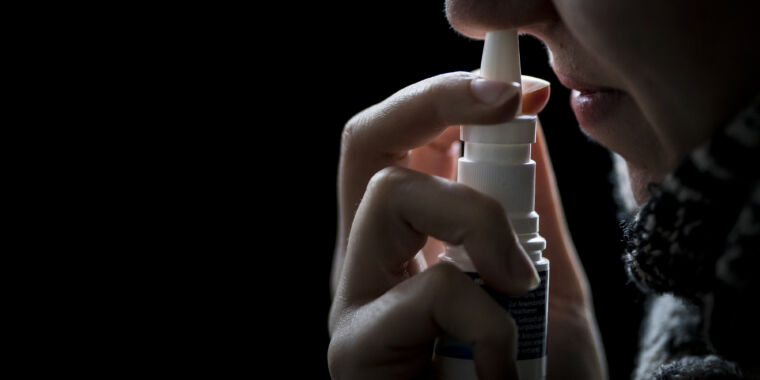
The maker of a homeopathic nasal spray with a history of contamination is refusing to recall its product after the Food and Drug Administration once again found evidence of dangerous microbial contamination.
In a warning Thursday, the FDA advised consumers to immediately stop using SnoreStop nasal spray—made by Green Pharmaceuticals—because it may contain microbes that, when sprayed directly into nasal cavities, can cause life-threatening infections. The FDA highlighted the risk to people with compromised immune systems and also children, since SnoreStop is marketed to kids as young as age 5.
According to the regulator, an FDA inspection in April uncovered laboratory test results showing that a batch of SnoreStop contained “significant microbial contamination.” But, instead of discarding the batch, FDA inspectors found evidence that Green Pharmaceuticals had repackaged some of the contaminated lot and distributed it as single spray bottles or as part of a starter kit.
In response, Green Pharmaceuticals destroyed the remainder of the tainted lot and stopped selling the nasal spray on its website. (It is still selling its SnoreStop throat spray, chewable tablets, and pet products, which includes a nasal spray.) But, according to the FDA, it refused to recall products that may contain product from the tainted lot. The agency said it “reiterated its recall recommendation multiple times” in August and September. But, “To date, the company has not taken action to recall this potentially dangerous product from the market.”
Ars has reached out to Green Pharmaceuticals for comment but has not received a response.
Tainted history
Enlarge / SnoreStop.
This isn’t new territory for the company. In 2022, Green Pharmaceuticals got warnings from the FDA and issued a recall due to microbial contamination in its SnoreStop nasal spray. In June 2022, the FDA held a conference with the company over findings of bacteria and fungi in the spray. Some of the results suggested high levels of microbial contamination. “The individual sample results varied between 420 and up to 6,200 colony forming units (CFU)/mL for total aerobic microbial count… and between 30 and up to 3,800 CFU/mL for total yeast and mold counts,” the FDA reported in a December 2022 warning letter sent after the fact.
The FDA also noted finding the specific bacterial pathogen Providencia rettgeri, an opportunistic germ that can lurk in health care settings. It’s most often linked to urinary tract infections, but it can also cause pneumonia, brain and spinal cord infections, heart infections, and wound and bloodstream infections in vulnerable people, according to a 2018 review.
“The high bioburden in conjunction with the route of administration with this drug product poses a high risk of harm to vulnerable patients, including children,” the FDA wrote in its warning letter. Green Pharmaceuticals recalled SnoreStop in June 2022, after its meeting with the FDA.
Dangerous dilutions
Aside from the gross microbial contamination, the FDA also noted in its letter that SnoreStop appears to be an unapproved new drug, illegally claiming to treat a disease without FDA approval. SnoreStop is a homeopathic product, meaning it is based on pseudoscience. Homeopaths falsely believe that if substances, including poisons, cause the same symptoms as illnesses, the substance can cure those illnesses (“like cures like”). The reason the products don’t poison users is because homeopaths also believe that diluting substances into oblivion enhances their curative properties (“law of infinitesimals”). Some dilutions are so extreme that not a single molecule of the starting substance is present in homeopathic products. And some homeopaths have argued that water molecules can have a “memory” of the substance, which, they contend, explains how the products work.
SnoreStop is said to contain dilutions of: nux vomica (a natural source of strychnine), belladonna (deadly nightshade), Ephedra vulgaris (a source of the drug ephedrine), hydrastis canadensis (a toxic herb), Kali Bichromicum (potassium dichromate, which is considered toxic and carcinogenic), Teucrium marum (similar to catnip), and Histaminum hydrochloricum (Histamine dihydrochloride).
Consumer advocates have worked for years to try to get homeopathic products off of store shelves, where they’re sometimes sold alongside evidence-based, FDA-approved over-the-counter medicines. While homeopathic products are mostly harmless and ineffective—offering placebo effects at best—they can turn deadly when manufacturers mishandle the dilutions. For instance, in 2016, the FDA linked improperly diluted belladonna in homeopathic teething products to the deaths of 10 infants and the poisonings of more than 400 others.
Homeopathic company refuses to recall life-threatening nasal spray, FDA says Read More »