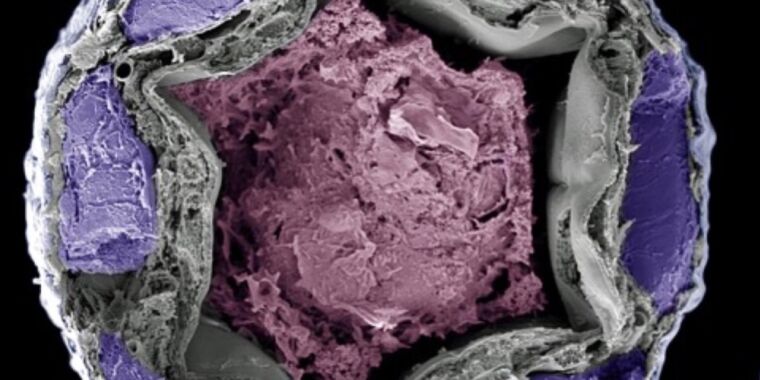
Welcome to “necroprinting”—3D printer nozzle made from mosquito’s proboscis
“To integrate the proboscis, we first removed it from an already euthanized mosquito under a microscope,” Cao explains. Then the proboscis/nozzle was aligned with the outlet of the plastic tip. Finally, the proboscis and the tip were bonded with UV-curable resin.
The necroprinter achieved a resolution ranging from 18 to 22 microns, which was two times smaller than the printers using the smallest commercially available metal dispensing tips. The first print tests included honeycomb structures measuring 600 microns, a microscale maple leaf, and scaffolds for cells.
But there were still areas in which human-made technology managed to beat Mother Nature.
Glass and pressure
The first issue with mosquito nozzles was their relatively low resistance to internal pressure. “It was impressive but still too low to accommodate some high viscosity inks,” Cao said.
These inks, which look more like a paste than a typical fluid, hold shape better, which translates into more geometrically accurate models that do not slump or spread under their own weight. This was a problem that Cao’s test prints experienced to an extent.
But this wasn’t the only area where human-made technology managed to beat nature. While mosquito nozzles could outperform plastic or metal alternatives in precision, they could not outperform glass dispensing tips, which can print lines below one micron across and withstand significantly higher pressures.
The researchers already have some ideas about how to bridge at least a part of this gap, though. “One possible solution is to use mosquito proboscis as the core and coat it with ceramic layers to provide much higher strength,” Cao said. And if the pressure problem is solved, the 18–22 microns resolution should be good enough for plenty of things.
Cao thinks that in the future, printers like this could be used to print scaffolds for living cells or microscopic electronic components. The idea is to replace expensive, traditional 3D printing nozzles with more affordable organic counterparts. The key advantages of mosquito nozzles, he says, are low cost and ubiquity.
Mosquitoes live almost everywhere on Earth and are easy to rear. The team estimates that organic 3D printing nozzles made from mosquito proboscises should cost around 80 cents; the glass and metal alternatives, the researchers state in the paper, cost between 32 and 100 times more.
“We already started doing more research on mosquitoes themselves and hope to develop more engineering solutions, not only to leverage their deceased bodies but also to solve practical problems they cause,” Cao said.
Science Advances, 2025. DOI: 10.1126/sciadv.adw9953
Welcome to “necroprinting”—3D printer nozzle made from mosquito’s proboscis Read More »