Big-name drugs see price drops in first round of Medicare negotiations
price cut —
If the prices were set in 2023, Medicare would have saved $6 billion.

Enlarge / Prescription drugs are displayed at NYC Discount Pharmacy in Manhattan on July 23, 2024.
In the first round of direct price negotiations between Medicare and drug manufacturers, prices for 10 expensive and commonly used drugs saw price cuts between 38 percent to 79 percent compared to their 2023 list prices, the White House and the US Department of Health and Human Services (HHS) announced Thursday. The new negotiated prices will take effect on January 1, 2026.
The 10 drugs that were up for negotiations are used to treat various conditions, from diabetes, psoriasis, blood clots, heart failure, and chronic kidney disease to blood cancers. About 9 million people with Medicare use at least one of the drugs on the list. In 2023, the 10 drugs accounted for $56.2 billion in total Medicare spending, or about 20 percent of total gross spending by Medicare Part D prescription drug coverage. But in 2018, spending on the 10 drugs was just about $20 billion, rising to 46 billion in 2022—a 134 percent rise. In 2022, Medicare enrollees collectively paid $3.4 billion in out-of-pocket costs for these drugs.
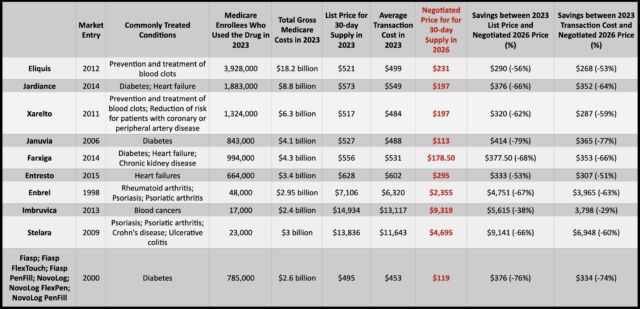
Enlarge / The 10 drugs as well as their use, 2023 costs, negotiated prices, and savings.
For now, it’s unclear how much the newly set prices will actually save those who have Medicare enrollees in 2026. Overall costs and out-of-pocket costs will depend on each member’s coverage plans and other drug spending. Additionally, in 2025, Medicare Part D enrollees will have their out-of-pocket drug costs capped at $2,000, which alone could significantly lower costs for some beneficiaries before the negotiated prices take effect.
If the newly negotiated prices took effect in 2023, HHS estimates it would have saved Medicare $6 billion. HHS also estimates that the prices will save Medicare enrollees $1.5 billion in out-of-pocket costs in 2026.
The price negotiations have been ongoing since last August when HHS announced the first 10 drugs up for negotiation. Medicare said it held three meetings with each of the drug manufacturers since then. For five drugs, the process of offers and counteroffers resulted in an agreed-upon price, with Medicare accepting revised counteroffers from drugmakers for four of the drugs. For the other five drugs, Medicare made final written offers on prices that were eventually accepted. If a drugmaker had rejected the offer, it would have either had to pay large fees or pull its drug from Medicare plans.
“The negotiations were comprehensive. They were intense. It took both sides to reach a good deal,” HHS Secretary Xavier Becerra told reporters Wednesday night.
“Price-setting scheme”
Both the price negotiations and the $2,000 cap are provisions in the Inflation Reduction Act (IRA), signed into law by President Biden in 2022. In a statement Thursday, Biden highlighted that Vice President Kamala Harris cast the tie-breaking vote to pass the legislation along party lines and that they are both committed to fighting Big Pharma. “[T]he Vice President and I are not backing down,” Biden said. “We will continue the fight to make sure all Americans can pay less for prescription drugs and to give more breathing room for American families.”
“Today’s announcement will be lifechanging for so many of our loved ones across the nation,” Harris said in her own statement, “and we are not stopping here.” She noted that the list of drugs up for Medicare negotiation will increase in each year, with an additional 15 drugs added in 2025.
In a scathing response to the negotiated prices, Steve Ubl—president of the industry group Pharmaceutical Research and Manufacturers of America (PhRMA)—called the negotiations a “price-setting scheme” and warned that patients would be disappointed. “There are no assurances patients will see lower out-of-pocket costs because the [IRA] did nothing to rein in abuses by insurance companies and PBMs who ultimately decide what medicines are covered and what patients pay at the pharmacy,” Ubl said. He went on to warn that IRA “fundamentally alters” the incentives for drug development and, as such, fewer drugs will be developed to treat cancer and many other conditions.
In a December 2023 report, the Congressional Budget Office estimated that “over the next 30 years, 13 fewer new drugs (of 1,300 estimated new drugs) will come to market as a result of the law.”
The pharmaceutical industry has unleashed a bevy of legal challenges to the negotiations, claiming they are unconstitutional. So far, it has lost every ruling.
Big-name drugs see price drops in first round of Medicare negotiations Read More »



