“Havard”-trained spa owner injected clients with bogus Botox, prosecutors say
Mounting evidence
Multiple clients and employees told investigators that Fadanelli also said she is a registered nurse, which is false. Though she is a registered aesthetician, aestheticians are not permitted to administer injections or prescription drugs.
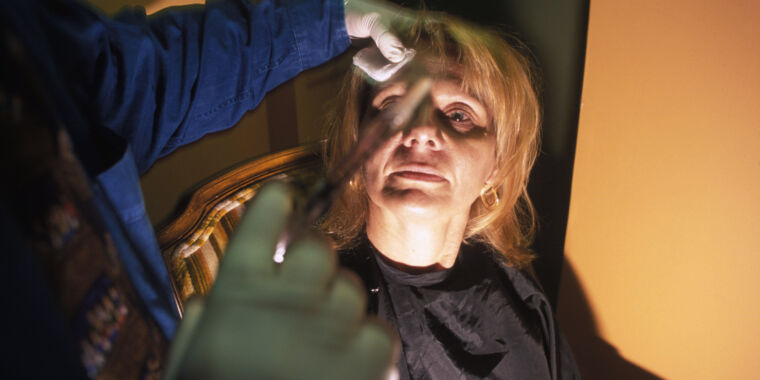
Investigators set up an undercover operation where an agent went in for a consultation, and Fadanelli provided a quote for a $450 Botox treatment. Investigators also obtained videos and images of Fadanelli performing injections. And the evidence points to those injections being counterfeit, prosecutors allege. Sales records from the spa indicate that Fadanelli performed 1,631 “Botox” injections, 95 “Sculptra” injections, and 990 injections of unspecified “filler,” all totaling over $933,000. But sales records from the manufacturers of the brand name drugs failed to turn up any record of Fadanelli or anyone else from her spa ever purchasing legitimate versions of the drugs.
Despite the mounting evidence against her, Fadanelli reportedly stuck to her story, denying that she ever told anyone she was a nurse and denying ever administering any injections. “When agents asked Fadanelli if she would like to retract or modify that claim if she knew there was evidence showing that she was in fact administering such products, she reiterated that she does not administer injections.”
Ars has reached to Fadanelli’s spa for comment and will update this story if we get a response. According to the affidavit, clients who received the allegedly bogus injections complained of bumps, tingling, and poor appearances, but no infections or other adverse health outcomes.
In a press release announcing her arrest, Acting United States Attorney for Massachusetts Joshua Levy said: “For years, Ms. Fadanelli allegedly put unsuspecting patients at risk by representing herself to be a nurse and then administering thousands of illegal, counterfeit injections. … The type of deception alleged here is illegal, reckless, and potentially life-threatening.”
For a charge of illegal importation, Fadanelli faces up to 20 years in prison and a $250,000 fine. For each of two charges of knowingly selling or dispensing a counterfeit drug or counterfeit device, she faces up to 10 years in prison and a fine of $250,000.
“Havard”-trained spa owner injected clients with bogus Botox, prosecutors say Read More »